Ask Lemmy
A Fediverse community for open-ended, thought provoking questions
Rules: (interactive)
1) Be nice and; have fun
Doxxing, trolling, sealioning, racism, and toxicity are not welcomed in AskLemmy. Remember what your mother said: if you can't say something nice, don't say anything at all. In addition, the site-wide Lemmy.world terms of service also apply here. Please familiarize yourself with them
2) All posts must end with a '?'
This is sort of like Jeopardy. Please phrase all post titles in the form of a proper question ending with ?
3) No spam
Please do not flood the community with nonsense. Actual suspected spammers will be banned on site. No astroturfing.
4) NSFW is okay, within reason
Just remember to tag posts with either a content warning or a [NSFW] tag. Overtly sexual posts are not allowed, please direct them to either !asklemmyafterdark@lemmy.world or !asklemmynsfw@lemmynsfw.com.
NSFW comments should be restricted to posts tagged [NSFW].
5) This is not a support community.
It is not a place for 'how do I?', type questions.
If you have any questions regarding the site itself or would like to report a community, please direct them to Lemmy.world Support or email info@lemmy.world. For other questions check our partnered communities list, or use the search function.
6) No US Politics.
Please don't post about current US Politics. If you need to do this, try !politicaldiscussion@lemmy.world or !askusa@discuss.online
Reminder: The terms of service apply here too.
Partnered Communities:
Logo design credit goes to: tubbadu
...galvanic reaction in this case, accelerated oxidation from contact between differential metals...
Thanks for the link.
That just doesn't explain the reaction between the two metals.
silver needs hydrogen sulfide to tarnish, although it may tarnish with oxygen over time. It often appears as a dull, gray or black film or coating over metal.
Somehow the silver got colourful instead of the usual grey.
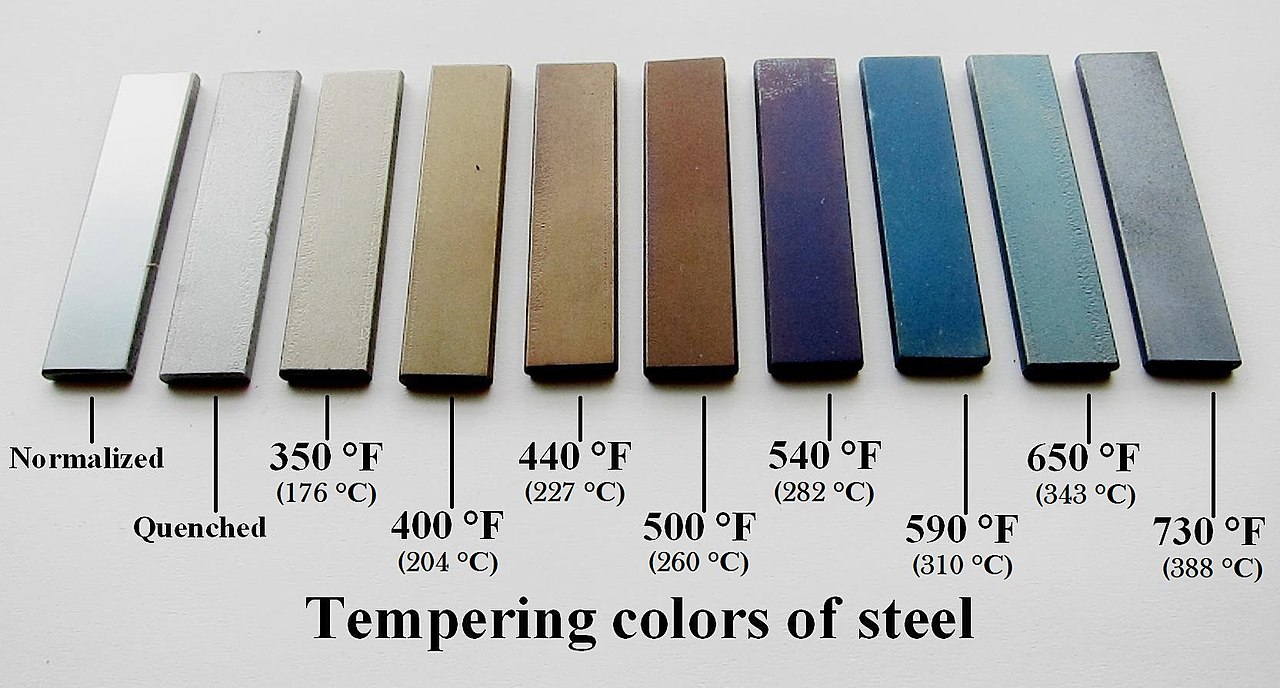
I recall reading something about titanium and its color. The thickness of the surface layer determines the color. It’s just nanometers thick, but that means light begins to do weird stuff at that scale. I suspect the same applies to the silver oxide/sulfide/whatever layer on the spoon. If that’s the case, you’re not actually seeing the color of the surface layer. The layer is exactly the right thickness that specific wavelengths of light get reflected back while others don’t.
Proper physicists can add more details.
Thanks!
Based on the phase interaction part of the article, there’s constructive and reconstructive phase interaction going on. That chapter has some nice diagrams about it.
That’s what produces the different colors. The thickness of the layer determines which wavelengths are lucky enough to reflect this way.
Also, here’s what happens with iron oxide.
There are multiple examples of the colorful toning in the article I linked. So you appreciated the link, but not enough ot actually look through it.
Pictures of tungsten tarnishing aren't helpful when op is talking about silver. Stating "thank you, that's the general idea yes, but I was trying to ask more specifically" isn't an attack on you. You don't need to defend yourself.
Right below the picture of tungsten is this picture of a silver dollar.

That is a surprisingly not blue tarnish, if its supposed to explain what OP is showing us, if it makes a point, please help me comprehend.
I do see that the light has mostly obscured a small band of blue so maybe its at a really neat phase of tarnish, but that's speculation and this photo provides no evidence that is actually the case. Either way, I really dont think the tarnish article goes far to actually explain the blue aside from describing the mechanism.
That is a surprisingly not blue tarnish this photo provides no evidence

Reading comprehension... What even is it?
I do see that the light has mostly obscured a small band of blue so maybe its at a really neat phase of tarnish, but that's speculation and this photo provides no evidence that is actually the case. Either way, I really dont think the tarnish article goes far to actually explain the blue aside from describing the mechanism.
You sandwiched your admission that the photo showed blue tarnish on silver between a claim that the photo didn't show blue and the false claim that the OP specifically asked why blue in particular.
Your post started with "pictures of tungsten aren't helpful". This is proof you didn't read the Wikipedia article where it shows a silver coin with tarnish.
The OP didn't say blue. They said colorful. You brought up blue to deflect from you not reading the article that showed colorful silver tarnish.
I read everything. (Even changed to my mother tongue.)
Didn't find anything about interactions of different metals. It only said something about interactions of metals and non-metals.
It's oxidation, so rust. You can clean it with silver polish.
To be pedantic, rust is explicitly iron oxide. Iron rusts, silver tarnishes.
Looks like the same kind of discoloration a steel spoon would get after heating. Like if you melted drugs using a lighter or blowtorch on it.
Fancy ass rich crack heads smoking with silver spoons.
Looking down on us, high on their horse...
or are they on their high horse? I hear white ponies are popular.
Sulfur causes that effect on silver, and it can happen slowly when exposed to humid air.
You can magically fix it by boiling water (must be boiling) then placing a sheet of aluminium foil in a container, placing the spoon on top of the foil making direct contact, them sprinkling some salt or sodium bicarbonate and pouring the boiling water on top. You'll see it start bubbling, and the bubbles will smell like rotten eggs.
Leave for fifteen minutes, you're done. But again, boiling water, this reaction requires a lot of energy and warm water won't even start it.
Did this recently with our silver. Worked fine with hot water (I used our kettle), just took a little longer. And I added the salt after the water and it still worked fine. But it's definitely one of those things where basic science feels like witchcraft when you do it lol
We had a plastic box with a plastic grid insert. We put the aluminum foil at the bottom, the grid on top of it to separate it from the silver, and added hot salt water. The cutlery needs to be moved around occasionally so every surface is treated evenly (and the water moves around).
Can confirm the sulphury smell, I usually did this out on the balcony.
And: the aluminum foil partially dissolves during this procedure, it looks like the moths got to it afterwards.
I remember seeing something, not unlike this in an episode of Mr. Wizard when I was a kid, lol
Generally it's silver reacting with (trace) amounts of sulfur containing gasses in the air to form silver sulfide.
Silver sulfide isn't blue, but the colours, and especially the multiple hues are caused by thin-film interference. That's the same effect that gives soap bubbles that shifting multi-colour effects.
The only way to remove it, is by grinding off a tiny bit of material, which is what you do when you polish it. The tarnish doesn't go further than the surface layer, so it's actually a pretty amazing way to preserve the metal.
Fun fact: if you ever want to make silver look old, put it in a closed box or bag with a hard boiled (or rotten, but I suggest boiled) egg. Those are high in sulfur compounds and will add months of tarnish in minutes.
I'd use a pencil eraser to polish off surface level tarnish, no need to bust out the grinders here. But otherwise your comment is on point.
He's not saying use a grinder. He's describing polishing as micro-grinding.
Technically correct, the best kind of correct.
This is why there was always a butler polishing the family silver.
Because they had no aluminium foil back then...
And back before that, expensive cutlery was made from aluminium.
Back when it was more expensive than gold, and people were not aware that this stuff was not the healthiest choice for coming in contact with food.
Something like this will fix 'er right up:

Silver needs to be polished every so often

Why did it spread from the magnet?

Not sure what exactly that means.

There's another magnet below to hold the other metal sign.
The stainless steel fridge looks fine.
The 800 means it's 80% silver. The other 20% are mostly copper.
The other symbols are maker's marks.
800 silver discolors faster than 925, but it's harder, cheaper and better for daily use.
it didn't spread from the magnet, it literally corroded everywhere except for the part of the surface under the magnet, which means the magnet shielded it from some environmental factor that caused the corrosion
Then how do you explain the bottom side? (And that the rest of the spoon looks regular silver /gray)
Serious question.
Edit: okay, I found that it starts at yellowing through reds then blues towards black. Can the back side have anything to do with the aluminum sign that hangs below?
And I still find it weird that there's some kind of aura around the magnets contact area.
The bottom side wear is probably largely wear from the spoon slightly wobbling every time the fridge door is opened and closed.
Regarding the magnet contact area, the magnet was blocking most of the ambient air from the silver. Given that the spoon was where most spoons live anyways, the kitchen, consider all the food scents and vapors it has experienced on the fridge, that likely accounts for the sulphur oxidation others have mentioned.
this multicolored pattern looks like this because thickness of layer of whatever is comparable to light wavelength, mechanism is the same as in oil layers on water being colorful. it didn't spread from magnet, the layer is thinnest near magnet and becomes thicker near edges, which suggest it might be just dirt/oils that accumulated on it by contact. try wiping it with alcohol or acetone or what have you
Could be caused by whatever coating is on the magnet, I tried to see if sulfur could be a component of a coating but gave up. Maybe others will just know.
That would make sense. I searched for neodymium coating and found that they're often coated with Nickel-Copper-Nickel or Zinc. 🤔